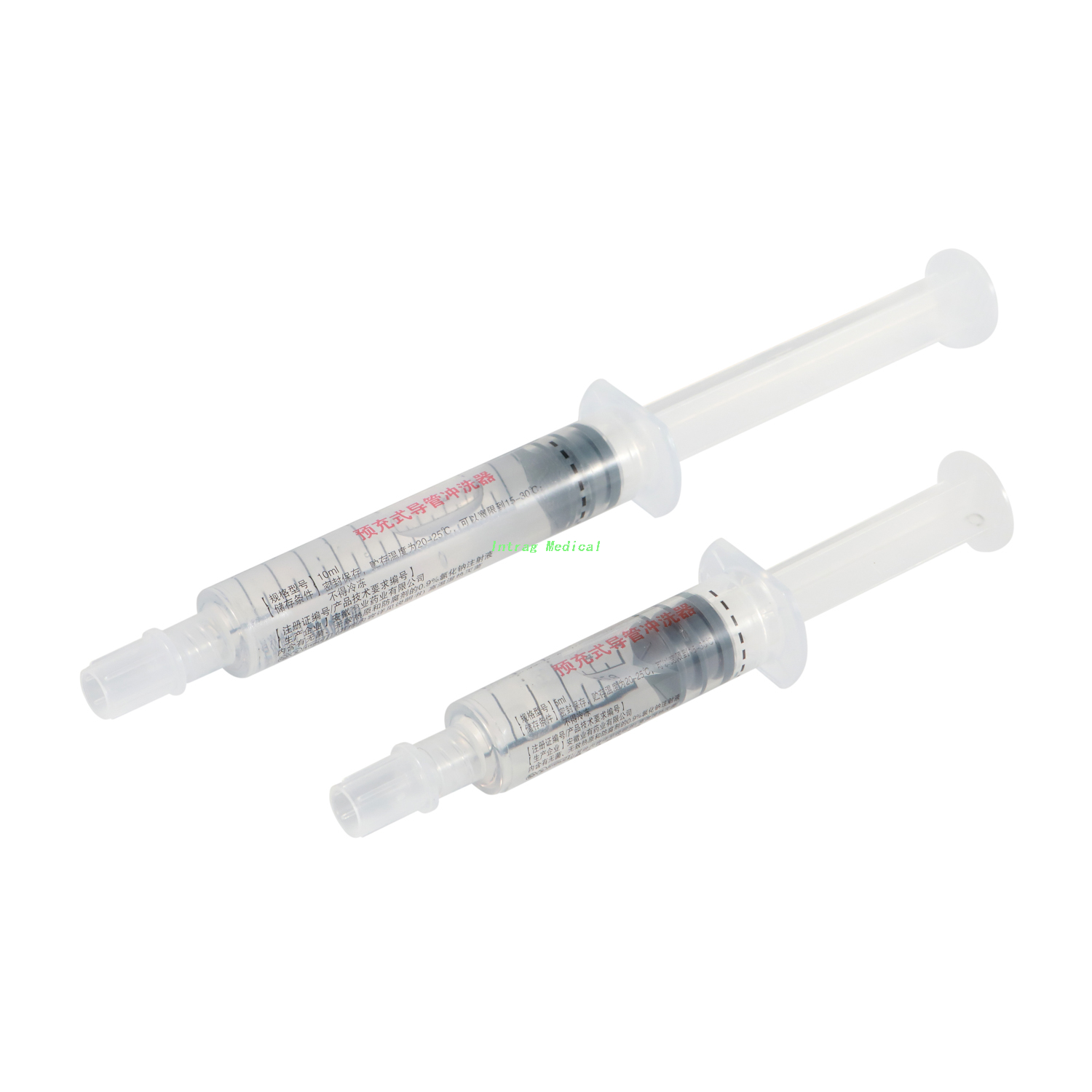
INTRAG’s Pre-Filled Normal Saline Flush Syringe is a ready-to-use medical device designed for flushing indwelling vascular access devices (IV catheters, PICC lines, central venous catheters). Pre-filled with 0.9% sodium chloride injection (USP standard), this three-piece syringe eliminates the need for manual filling, reducing preparation time and the risk of contamination. Available in 3ml, 5ml, and 10ml capacities, it is a critical tool for maintaining vascular access patency in hospitals, long-term care facilities, and home healthcare.
| Availability: | |
|---|---|
| Quantity: | |











3ml,5ml,10ml
INTRAG
The syringe consists of a barrel, plunger, piston, nozzle cap, and pre-filled 0.9% sodium chloride solution. The barrel is made from high-clarity polypropylene, allowing visual inspection of the solution. The 6% luer connector ensures compatibility with standard vascular access devices, and the tip cap maintains sterility until use. The solution is isotonic and sterile, conforming to USP standards for purity and pH balance.
The pre-filled design saves healthcare providers time, especially in busy clinical settings, and reduces the risk of medication errors (e.g., incorrect dilution). The sterile fluid path, sterilized via moist heat, ensures the solution remains free from contaminants. The syringe is lightweight and easy to handle, with a smooth plunger that allows controlled flushing without sudden pressure spikes.
The global pre-filled flush syringes market is growing at a CAGR of 7.3%, driven by the increasing use of indwelling vascular devices, rising focus on infection control, and the need to streamline clinical workflows. Hospitals are the largest end-users, with long-term care facilities and home healthcare services emerging as fast-growing segments. INTRAG’s pre-filled syringe addresses key pain points (contamination risk, preparation time) for healthcare providers, making it a valuable addition to clinical supply chains. The 3ml, 5ml, and 10ml capacities cater to different vascular access types, from peripheral IVs (3ml) to central lines (10ml).
Parameter | Specification |
Model | 3ml, 5ml, 10ml |
Solution | 0.9% sodium chloride injection (USP standard) |
Construction | 3-piece (barrel, plunger, piston) |
Connector Type | 6% luer connector |
Sterilization Method | Moist heat sterilization |
Package Type | Individual blister pack |
Shelf Life | 3 years from manufacture date |
pH Range | 4.5-7.0 (conforms to USP) |
Osmolarity | 280-310 mOsmol/L |
Applications | Flushing indwelling vascular access devices (IV catheters, PICC lines, central venous catheters) |
Single-Use | Yes |
Pre-Filled Convenience: Ready-to-use 0.9% sodium chloride solution eliminates manual filling, saving time and reducing contamination risk.
Sterile Fluid Path: Moist heat sterilization ensures the solution and syringe interior are free from bacteria, viruses, and pyrogens.
Compatibility: 6% luer connector fits all standard vascular access devices, ensuring universal use in clinical settings.
Clear Visual Inspection: Transparent barrel allows verification of solution clarity and volume before use.
Long Shelf Life: 3-year shelf life when stored properly, ensuring inventory flexibility for healthcare facilities.
The Pre-Filled Normal Saline Flush Syringe complies with USP <788> (Particulate Matter in Injections) and ISO 13485 (Quality Management Systems). It meets CE and FDA requirements for pre-filled medical devices, and the 0.9% sodium chloride solution adheres to USP standards for purity, pH, and osmolarity. The syringe is tested for leak resistance and flow consistency, ensuring reliable performance in critical clinical applications.
Pre-filled syringes reduce the risk of contamination (from manual handling of saline bags and syringes) and medication errors (e.g., incorrect dilution). They also save time, allowing healthcare providers to focus on patient care rather than preparation.
Store in unopened blister packs at room temperature (15°C-30°C) away from direct sunlight and extreme temperatures. Do not freeze the syringe, as this may damage the solution or syringe structure.
No, this syringe is specifically designed for flushing vascular access devices. The 0.9% sodium chloride solution is isotonic but not intended for therapeutic injection (e.g., hydration). Use only as directed for flushing.
Do not use the syringe if the solution is cloudy, discolored, or contains particles. This may indicate contamination. Dispose of the syringe properly and use a new one. Always inspect the solution before connecting to a vascular access device.
The syringe consists of a barrel, plunger, piston, nozzle cap, and pre-filled 0.9% sodium chloride solution. The barrel is made from high-clarity polypropylene, allowing visual inspection of the solution. The 6% luer connector ensures compatibility with standard vascular access devices, and the tip cap maintains sterility until use. The solution is isotonic and sterile, conforming to USP standards for purity and pH balance.
The pre-filled design saves healthcare providers time, especially in busy clinical settings, and reduces the risk of medication errors (e.g., incorrect dilution). The sterile fluid path, sterilized via moist heat, ensures the solution remains free from contaminants. The syringe is lightweight and easy to handle, with a smooth plunger that allows controlled flushing without sudden pressure spikes.
The global pre-filled flush syringes market is growing at a CAGR of 7.3%, driven by the increasing use of indwelling vascular devices, rising focus on infection control, and the need to streamline clinical workflows. Hospitals are the largest end-users, with long-term care facilities and home healthcare services emerging as fast-growing segments. INTRAG’s pre-filled syringe addresses key pain points (contamination risk, preparation time) for healthcare providers, making it a valuable addition to clinical supply chains. The 3ml, 5ml, and 10ml capacities cater to different vascular access types, from peripheral IVs (3ml) to central lines (10ml).
Parameter | Specification |
Model | 3ml, 5ml, 10ml |
Solution | 0.9% sodium chloride injection (USP standard) |
Construction | 3-piece (barrel, plunger, piston) |
Connector Type | 6% luer connector |
Sterilization Method | Moist heat sterilization |
Package Type | Individual blister pack |
Shelf Life | 3 years from manufacture date |
pH Range | 4.5-7.0 (conforms to USP) |
Osmolarity | 280-310 mOsmol/L |
Applications | Flushing indwelling vascular access devices (IV catheters, PICC lines, central venous catheters) |
Single-Use | Yes |

Pre-Filled Convenience: Ready-to-use 0.9% sodium chloride solution eliminates manual filling, saving time and reducing contamination risk.
Sterile Fluid Path: Moist heat sterilization ensures the solution and syringe interior are free from bacteria, viruses, and pyrogens.
Compatibility: 6% luer connector fits all standard vascular access devices, ensuring universal use in clinical settings.
Clear Visual Inspection: Transparent barrel allows verification of solution clarity and volume before use.
Long Shelf Life: 3-year shelf life when stored properly, ensuring inventory flexibility for healthcare facilities.
The Pre-Filled Normal Saline Flush Syringe complies with USP <788> (Particulate Matter in Injections) and ISO 13485 (Quality Management Systems). It meets CE and FDA requirements for pre-filled medical devices, and the 0.9% sodium chloride solution adheres to USP standards for purity, pH, and osmolarity. The syringe is tested for leak resistance and flow consistency, ensuring reliable performance in critical clinical applications.
Pre-filled syringes reduce the risk of contamination (from manual handling of saline bags and syringes) and medication errors (e.g., incorrect dilution). They also save time, allowing healthcare providers to focus on patient care rather than preparation.
Store in unopened blister packs at room temperature (15°C-30°C) away from direct sunlight and extreme temperatures. Do not freeze the syringe, as this may damage the solution or syringe structure.
No, this syringe is specifically designed for flushing vascular access devices. The 0.9% sodium chloride solution is isotonic but not intended for therapeutic injection (e.g., hydration). Use only as directed for flushing.
Do not use the syringe if the solution is cloudy, discolored, or contains particles. This may indicate contamination. Dispose of the syringe properly and use a new one. Always inspect the solution before connecting to a vascular access device.